Clinical Trials: What to know
Clinical trials ensure that safe, effective medicines make it to the people who need them.
What is a clinical trial?
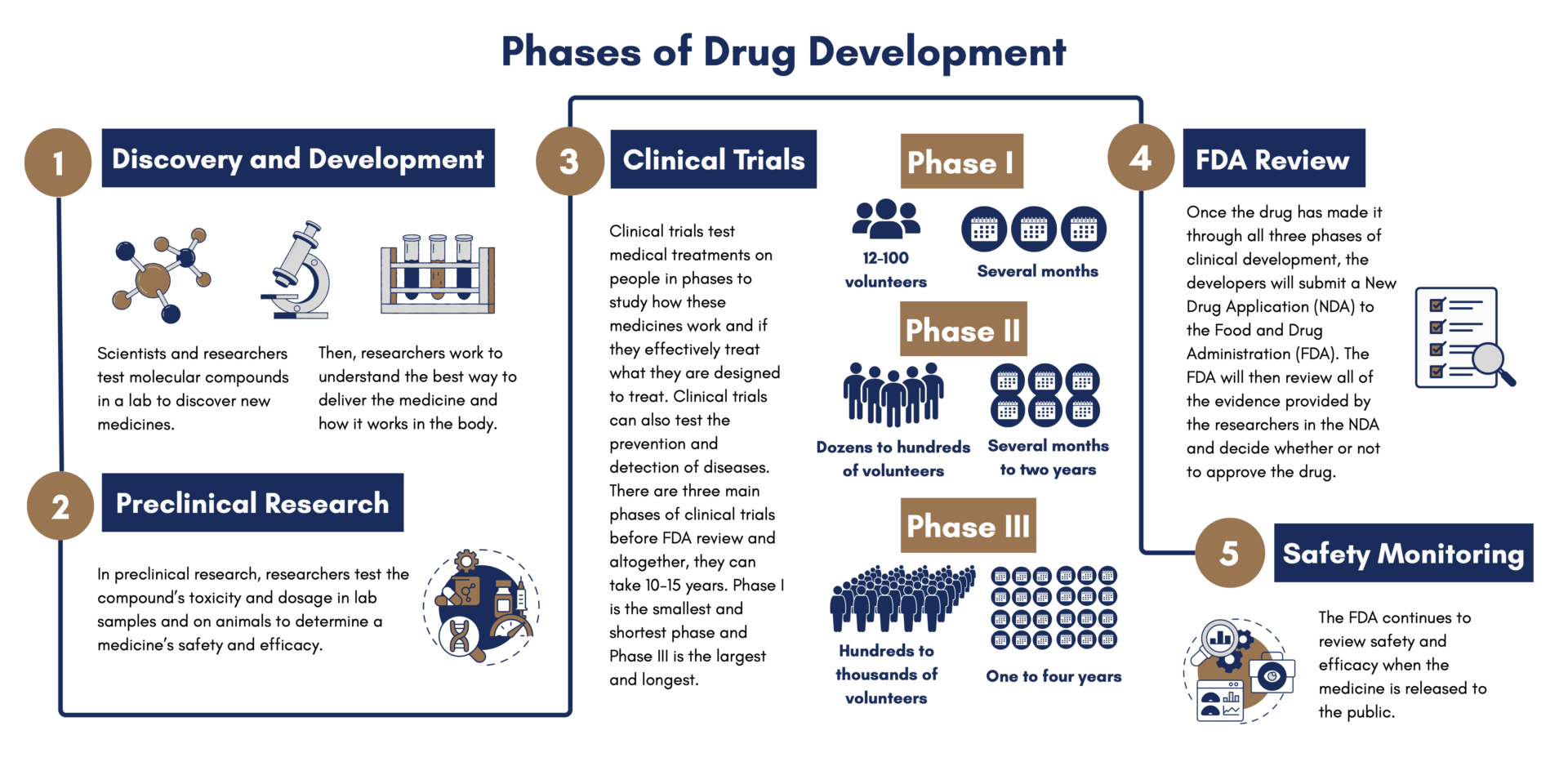
Clinical trials are just one of the many steps involved in getting a medicine from the lab and into patients’ hands. First, in the discovery and development stage, scientists and researchers test molecular compounds, and then, in the preclinical stage, researchers test the compound’s toxicity and dosage on animals. Clinical trials test medical treatments on people in phases to study how these medicines work and if they effectively treat what they are designed to treat. Clinical trials can also test the prevention and detection of diseases.
Only about 8% of investigational new drugs that begin clinical testing make it through all stages and reach regulatory approval. In some cases, the process can take about 10-15 years, though for rare diseases like cystic fibrosis, drugs can move faster.
For drug makers, it is a time and resource intensive process. For the people who enroll in clinical trials, often referred to as participants, it is often a deeply personal decision to do so. For researchers and clinicians who rely on clinical trials to advance the science about a given medical condition, clinical trials represent the final part of the drug development process that brings new medicines from the test tube to the patient.
What are the phases of clinical trials?
Phase 1 – This phase includes several dozen to one hundred volunteers and takes several months to complete. About 70% of drugs move through this phase. The goal of this phase is to learn about the drug’s safety and side effects. Sometimes, these participants are “normal healthy volunteers” rather than people who live with a given medical condition. Other times, patients themselves can be included in phase I trials.
Phase 2 – This phase includes many dozen to several hundred people and can last from several months to two years. About one third of drugs move through this phase. The goal of this phase is to see how effective the treatment is and to continue to monitor its safety.
Phase 3 – This phase can include up to several hundred to thousands of volunteers and can last one to four years. About one quarter of drugs make it through this phase. The goal of this phase is to confirm how effective the drug is and to compare it with other treatments.
If Phase 3 is successful, drugs can be approved by the FDA.
Phase 4 – This phase continually monitors safety and efficacy after the FDA approves the drug and it becomes publicly available.
Are clinical trials safe?
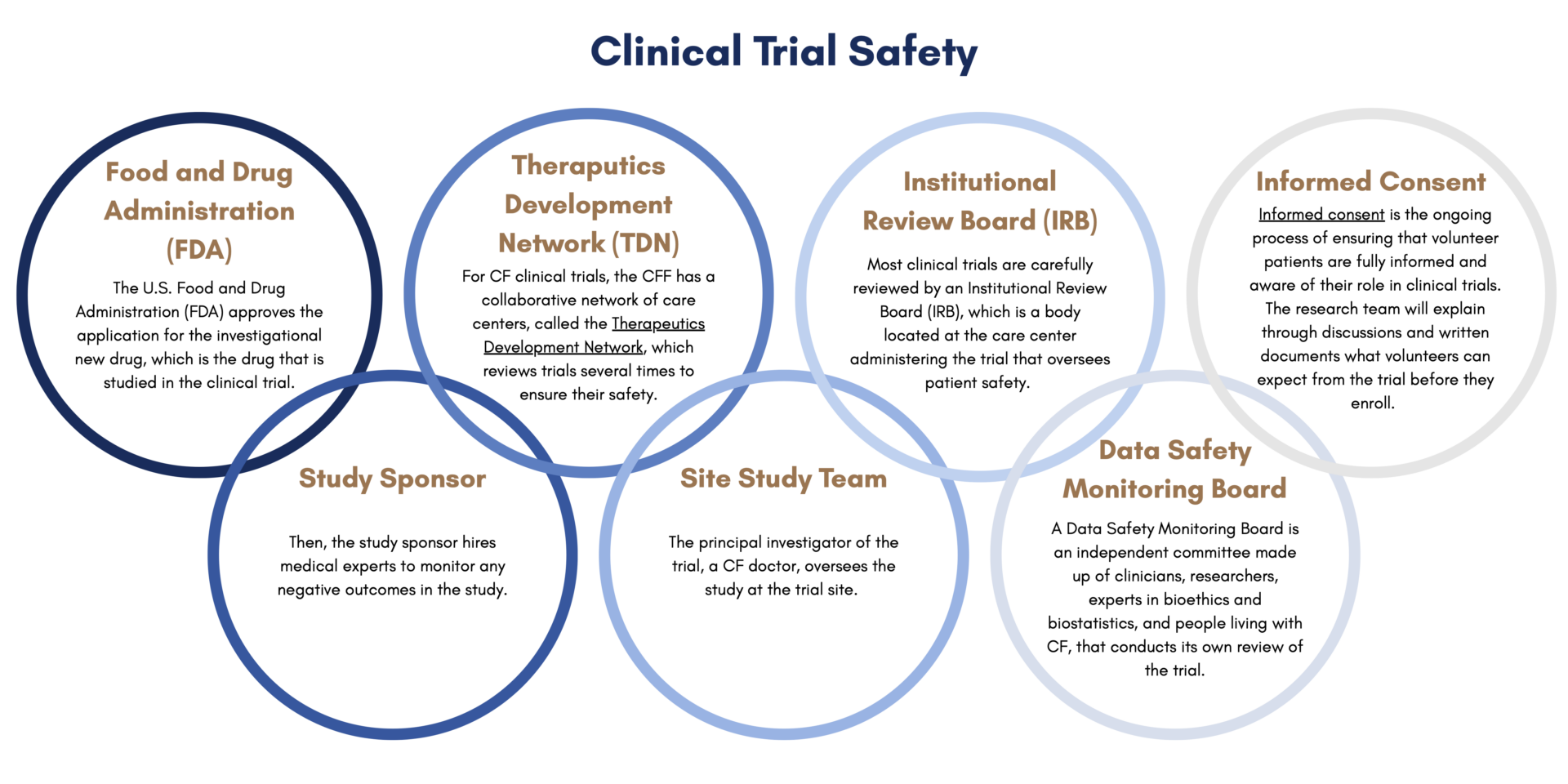
Before patients start a clinical trial, they go through a process called informed consent. Informed consent is the ongoing process of ensuring that volunteer patients are fully informed and aware of their role in clinical trials. The research team will explain through discussions and written documents what volunteers can expect from the trial before they enroll. This includes what the purpose of the trial is, how long it is projected to last, what is required to participate, and the potential risks and benefits.
There are many layers of safety built into the clinical trial process. First, the U.S. Food and Drug Administration (FDA) approves the application for the investigational new drug, which is the drug that is studied in the clinical trial. Then, the study sponsor hires medical experts to monitor any negative outcomes in the study. For CF clinical trials, the Cystic Fibrosis Foundation has a collaborative network of care centers, called the Therapeutics Development Network, which reviews trials several times to ensure their safety. Clinical trials also have a Data Safety Monitoring Board, which is an independent committee made up of clinicians, researchers, experts in bioethics and biostatistics, and people living with CF, that conducts its own review of the trial. Most clinical trials are carefully reviewed by an Institutional Review Board (IRB), which is a body located at the care center administering the trial that oversees patient safety. The principal investigator of the trial, a CF doctor, oversees the study at the trial site. CFF has a graphic that explains how these layers of safety work together.
What can I expect from participating in a clinical trial?
Every clinical trial is different depending on the treatment that is being tested. Before enrolling in a clinical trial, patients will have the opportunity to review the trial’s protocol, which is a document that explains what will go on during the trial and why. The protocol will list all of the relevant information regarding participation, including how long the trial will last and what volunteers are expected to do.
How do I find a clinical trial?
For CF-specific clinical trials, the Cystic Fibrosis Foundation has a comprehensive resource called the Clinical Trial Finder that lists active CF clinical trials. The Clinical Trial Finder lists trials for genetic therapies, anti-infectives, nutrition and GI, observational studies, and more.
Emily’s Entourage also has a Clinical Trial Connect for clinical trials specifically for people living with CF who do not benefit from modulators.
FAQS
The eligibility criteria for a clinical trial will be clearly listed on the trial protocol, which is a document that explains what will go on during the trial and why. Eligibility criteria can include age, type of CFTR mutation, health status, treatment status, and what other conditions the patient has.
Sponsors, such as pharmaceutical companies, academic institutions, government agencies, and nonprofits, provide funding for clinical trials.
Clinical trials are completely voluntary and volunteers can leave at any time.
